HUBLE METHODS
Ex Vivo Bone-Myeloma Organ Cultures
Full Text
PDF
Full Text
Introduction
This HubLE Method describes the protocol to establish ex vivo co-cultures between bone tissue and myeloma cancer cells to preserve the 3-dimensional distribution and cellularity of the bone/bone marrow microenvironment.
Materials
- Mice (7 to 9 days old) [Tip No.1]
- Forceps and scissors
- 5mm biopsy hole punch
- 24-well plate
- Sterile phosphate-buffered saline (PBS)
- aMEM tissue culture medium (10% FBS, Penicillin and Streptomycin)
- 96-well plate (2x)
- 37˚C and 5% C02cell incubator
- Myeloma cells
Methods [Update]
- Day 1, dissect the calvarial bones, clean muscle and blood, and transfer to a 24-well plate filled with sterile cold PBS.
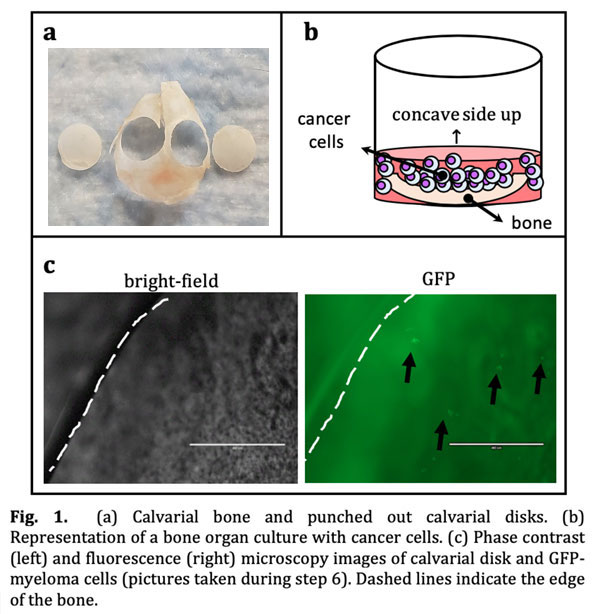
- Punch 2 holes in each calvaria using a 5mm biopsy punch, avoiding cranial sutures. (Fig. 1a)
- Transfer 5mm calvaria disks, concave side up, to a 96-well plate containing 200μL of media per well. Culture overnight.
- Day 2, prepare a cell suspension of 50,000 5TGM1 myeloma cells per 50μl. [Tip No.2]
- Remove media in the plate and add 50L (50,000 myeloma cells) of the cell suspension slowly to the center of the calvarial disk and incubate at 37˚C for 24h. [Tip No.3]
- Day 3, transfer calvarial disks with myeloma cells to a new 96-well plate. [Tip No.4]
- Fill wells with 200μL of media and incubate at 37˚C and 5% CO2. Refresh half of the media every 48h. Keep cultures up to 12 days.
- Collect conditioned media for assessing secreted protein levels and calvarial disks for gene expression. [Tip No.5]
Tips [Update]
- Ex vivobone organ cultures can be established with calvarial disks obtained from either immunodeficient (C57BL/SCID-/-) or immunocompetent (C57BL/KaLwRij)female/male mice.
- We use 50mL of the cell suspension to ensure myeloma cells are in close contact with the bone tissue. Locate the concave side of the calvarial disks up to facilitate retention of myeloma cells. Human myeloma cells lines (JJN3, MM1.S) can also be used in these cultures (Fig. 1b).
- We recommend adding the cell suspension to the calvarial disk immediately after media removal to ensure disks do not dry out.
- This step is required to discard myeloma cells that did not attached/engrafted into the bone tissue and accumulate in edge of the wells without directly interacting with calvarial disks. We recommend using forceps placed close to the edge of the calvarial disks to transfer them to new plates.
- We recommend collecting conditioned media at different time points to assess the levels of secreted proteins and circulating markers (bone resorption, bone formation, tumor burden), and harvest calvarial disks for RNA/protein isolation and/or histology.
References [Update]
- Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N et al. Bidirectional Notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res 2016; 76(5):1089-1100.
- Delgado-Calle J, Kurihara N, Atkinson EG, Nelson J, Miyagawa K, Galmarini CM, Roodman GD, Bellido T. Aplidin (plitidepsin) is a novel anti-myeloma agent with potent anti-resorptive activity mediated by direct effects on osteoclasts. Oncotarget 2019; 10(28):2709-2721.
- Delgado-Calle J, Hancock B, Likine EF, Sato AY, McAndrews K, Sanudo C et al. MMP14 is a novel target of PTH signaling in osteocytes that controls resorption by regulating soluble RANKL production. FASEB J 2018; 32(5):2878-2890.
- Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N et al. Osteocytes mediate the anabolic actions of canonical Wnt/b-catenin signaling in bone. Proc Natl Acad Sci U S A 2015; 112(5):E478-E486.
PDF

Adam Ferrari, MSc
Department of Medicine
Division of Hematology/
Oncology, Department of Anatomy and Cell Biology
Email: aferrari.med@gmail.com

Jesus Delgado-Calle, PhD
Department of Medicine
Division of Hematology/
Oncology, Department of Anatomy and Cell Biology
Email: jedelgad@iupui.edu