HUBLE METHODS
Isolation and culture of primary rodent osteoblasts
Introduction
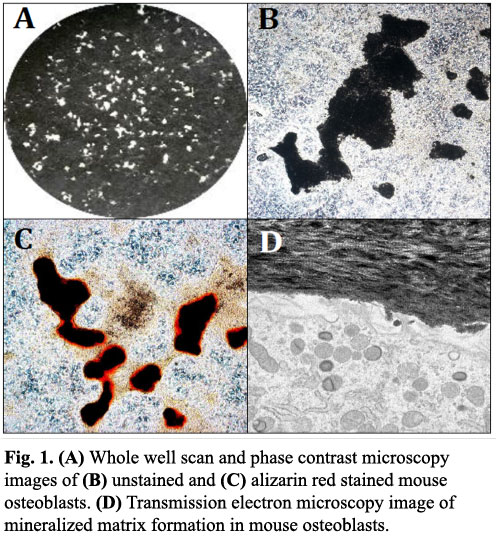
The in vitro culture of calvarial osteoblasts from neonatal rodents represents a widely used technique for studying osteoblast function (Fig. 1)[1,2]. This HubLE Method describes the protocol for isolation and culture of these cells.
Materials
- Animals [Tip No. 1]
- Phosphate buffered saline (PBS)
- Alpha modified essential medium (αMEM) [Tip No. 2]
- Osteogenesis αMEM (osMEM) [Tip No. 3]
- Trypsin-EDTA (0.25%)
- Type II collagenase from Clostridium histolyticum (0.2%)
- Glutaraldehyde (2.5%)
- Alizarin Red (1%)
Methods [Update]
- Carefully isolate the calvaria, taking care to remove all excess tissue and cartilage.
- Cut in half and place in a flat bottomed tube, rinse with phosphate buffered saline (PBS).
- Incubate in 500ml of 0.25% trypsin for 10 minutes at 37°C.
- Wash with αMEM, discard solution
- Incubate in 500ml of 0.2% type II collagenase for 30 minutes at 37° Steps 3-5 remove non-osteoblastic cells from the sample.
- Remove collagenase, discard and replace with fresh collagenase solution for a further 60 minutes.
- Keep the final digest and spin at 1,500g for 5 minutes.
- Resuspend in 1ml αMEM per calvaria and transfer cell suspension to 75cm2 flask containing αMEM. Rat cells = 1 calvaria/flask, Mouse cells = 3 calvaria/flask.
- Incubate at 37°C/5% CO2 until confluent (~ 4 days).
- Once confluent, remove medium, wash with PBS and incubate with 0.25% Trypsin for 5-10 minutes to detach cells.
- Spin at 1,500g for 5 minutes and resuspend in αMEM (1ml per flask).
- Perform a cell count and seed cells in tissue culture trays in osMEM [Tip No. 4 & No. 5].
- Culture for up to 21 days with half medium changes every 2-3 days.
- Fix with 2.5% glutaraldehyde for 5 minutes before staining with 1% alizarin red to visualise mineralized nodules [Tip No.6].
Tips [Update]
Animals: cells can be obtained from neonatal rats (2-3 days) or mice (3-6 days). After expansion, the following cell yields per calvaria can be expected: rat (~7×106 cells) and mouse (~4×106).
αMEM: Add 10% foetal calf serum (FCS) and AB/AM (100U/ml penicillin, 100mg/ml streptomycin, 0.25mg/ml amphotericin). Rat cells can also be cultured in Dulbecco’s modified essential medium (DMEM) supplemented with 10% FCS, AB/AM and 2mM L-glutamine.
Osteogenesis αMEM: to add αMEM add 50mg/ml ascorbate, 2mM β-glycerophosphate and, for rat cells only, 10nM dexamethasone. Always make fresh on the day of use.
Tissue culture plates: Rat osteoblasts can be cultured successfully in a number of well plate formats, whilst mouse osteoblasts will only form abundant mineralized nodules in 12 or 6 well plates. Culture in 24 well trays will result in significant peeling prior to mineralization. Typical seeding densities per well: 2.5×104 (24-well), 5×104 (12-well), 105 (6-well). Plate all cells at this stage: do not passage primary osteoblasts as it will result in a significant loss of phenotype leading to longer cultures and delayed, if any, mineralization.
β-glycerophosphate: This method uses a low concentration of β-glycerophosphate (2mM) since culture with 5-10mM results in reduced cell viability, alkaline phosphatase expression and causes widespread, non-specific mineral deposition that differs from true bone formation.
Alizarin Red staining: Mineralised bone nodules can be stained with alizarin red; however, it is also possible to perform image analysis and obtain good quality images on unstained cell layers (Fig 1A-1B).
References [Update]
1. Perpetuo IP, Bourne LE, Orriss IR (2019). Isolation and generation of osteoblasts. Methods Mol Biol, 1914:21-38.
Orriss IR, Hajjawi MOR, Heusa C, MacRae V, Arnett TR (2014). Optimisation of the differing conditions required for bone formation in vitro by primary osteoblasts from mice and rats. In J Mol Med, 34:1201-1208.

Lucie E Bourne, BSc
Department of Comparative Biomedical Sciences,
Royal Veterinary College, London. NW1 0TU, UK.
Email: lbourne3@rvc.ac.uk

Isabel R Orriss, BSc, PhD
Department of Comparative Biomedical Sciences,
Royal Veterinary College, London, NW1 0TU, UK
Email: iorriss@rvc.ac.uk