HUBLE METHODS
Isolation, generation and culture of bone marrow derived mouse osteoclasts
Introduction
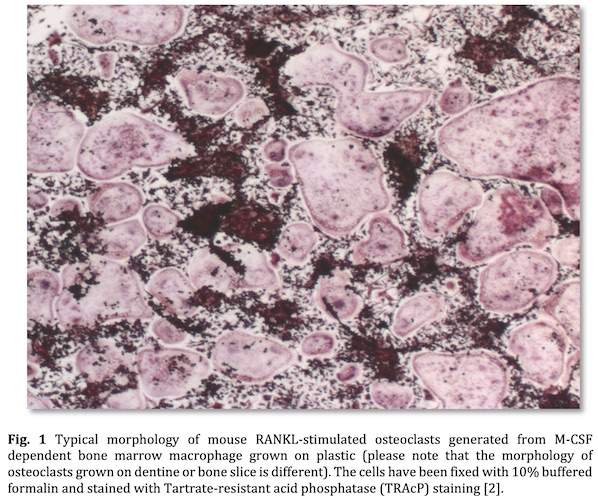
The in vitro culture of mouse osteoclasts is a widely used technique for studying osteoclast formation, survival and function (Fig. 1) [1]. This HubLE Method describes the protocol for isolation, generation and maintenance of RANKL generated osteoclasts from M-CSF dependent bone marrow macrophage.
Materials
- Recombinant Receptor activator of nuclear factor-κB ligand (rRANKL)
- Recombinant Monocyte colony stimulating factor (rM-CSF)
- Penicillin/Streptomycin
- Foetal bovine serum (FBS)
- Calf serum (CS)
- Complete media (CM): Minimum Essential Medium Eagle – Alpha modification (αMEM) supplemented with penicillin (100U/mL) and streptomycin (100µg/mL) + 2.5%FBS + 2.5%CS
- Sterile Petri dish (100mm)
- Centrifuge tube (15 ml)
- Phosphate buffered saline (PBS)
Methods [Update]
(A) Generation of bone marrow macrophage
- Dissect and remove skin, paws and muscle from the long bones (tibia and femur) of 8–12- week-old mice, and place in a sterile Petri dish (100mm) containing ice cold PBS.
- Cut the epiphyses and flush out the bone marrow using a 5ml syringe with a 25-gauge (G) needle [Tip 1].
- Centrifuge the single cell suspension at 300 g for 3 minutes, discard the supernatant and resuspend the pellet in complete media (CM)[Tip 2].
- Culture cell suspension in CM supplemented with rM-CSF (100ng/ml) for 72 hours [Tip 3]
(B) Generation of multinucleated osteoclasts
- Gently scrape the adherent mono-layer of rM-CSF dependent macrophages into CM, transfer to a conical 15ml tube and centrifuge the tube at 300 g for 3 minutes.
- Discard the supernatant, resuspend the pellet in CM [Tip 2], and estimate cell number using a Hemocytometer.
- Seed cells at a density ranging from 103 to 105 cells/cm2 in desired tissue culture plate in CM supplemented with rM-CSF (25ng/ml) and rRANKL (10–120 ng/ml) [Tip 4].
- Refresh 50% of CM supplemented with rM-CSF (25ng/ml) and rRANKL (10–120 ng/ml) every 48h until multi-nucleated osteoclasts are formed; typically from 5 to 7 days of stimulation are needed [Tip 5]
Tips [Update]
To obtain a single cell suspension resuspend using needles of decreasing size (19G-21-25G).
Resuspended cell suspension from 1 mouse in 1ml of culture medium.
Wash non-adherent cells and debris with 5ml ice-cold PBS after 16 – 24 hours and then incubate adherent cell in CM supplemented with rM-CSF (25–50ng/ml) until confluent M-CSF-dependent macrophages are obtained (approximately 48 -72 hours).
Please refer to manufacturer’s instructions for the concentration of rRANKL to be used or vary the concentration used until the desired osteoclast number is obtained.
Mature osteoclasts should be visible under standard microscope prior TRAcP staining [2].
Osteoclast bone resorption can be analysed in vitro if cells are cultured on dentine or bone slices
References [Update]
Marino S, Logan JG, Mellis D, & Capulli M (2014). Generation and culture of osteoclasts. BoneKEy reports 3: 570.
Idris, A.I., van’t Hof, R. TRAcP staining, HubLE Methods. DOI: 10.13140/RG.2.2.25993.90720.

Silvia Marino, PhD
Indiana University,
Division of Haematology/Oncology, Indianapolis. USA.
Email: simarino@iu.edu

Mattia Capulli, PhD
Univeristy of L’ Aquila,
Deparment of Biotechnological and Applied Clinical Sciences, L’ Aquila, Italy
Email: mattia.capulli@univaq.it

