IN A NUTSHELL
Enhanced Arginase-1 Ameliorates Arthritic Inflammation
Rheumatoid arthritis is a chronic inflammatory disorder of the joints affecting up to 1 % of the global population. Rheumatoid arthritis patients suffer from synovial inflammation in the joints including immune cell infiltration, cartilage and bone destruction. Macrophages in particular have a pivotal role due to their widespread pro-inflammatory, destructive and remodeling capacities that can critically contribute to disease symptoms.
Their precarious responses require transcriptional adjustment and refinement, resulting in a spectrum of macrophage subsets, each specific in terms of their stimulation. Therefore, their activation constitutes a transcriptional reprogramming, which involves the cooperation of several transcription factors. One of these transcription factors is the activator protein (AP) -1 family.
Due to their sophisticated actions, existing evidence on AP-1 functions is limited and their target genes are not yet completely known. Consequently, the aim of our study (https://www.jci.org/articles/view/96832) was to investigate novel AP-1 functions in macrophages, particularly the AP-1 family member Fra-1. Our study demonstrates that Fra-1 skewed macrophage action towards a pro-inflammatory phenotype, suppressing pro-resolving actions through the enzyme arginase-1 (Arg1). We identified Fra-1 as a key switch regulator of macrophage function, skewing the macrophage phenotype towards a pro-inflammatory state, thereby promoting the arthritic joint inflammation.
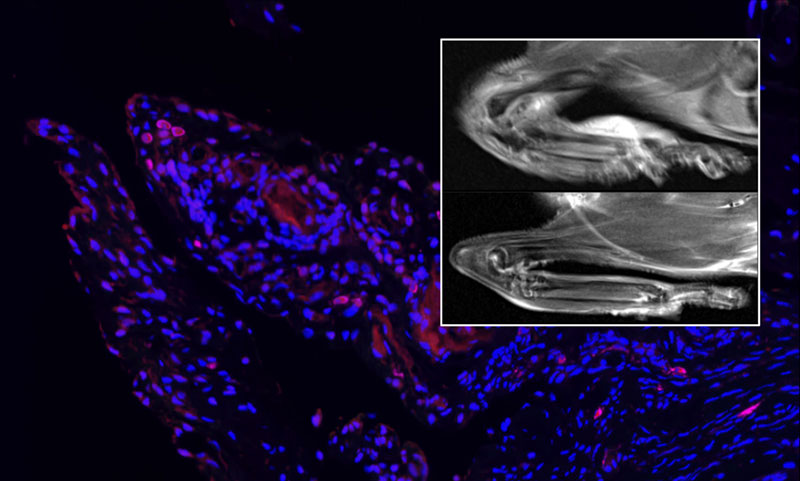
Arg1 is a hallmark of anti-inflammatory macrophages and drives macrophage pro-resolving functions, which initiate the resolution of inflammation and restoration of tissue homeostasis. Indeed, in animal arthritis models we were able to demonstrate that inhibited Arg1 activity worsened arthritic symptoms. Remarkably, enhanced Arg1 activity, through supplementation with its substrate arginine, led to an ameliorated arthritic inflammation with reduced bone erosion. Therefore, we assume that macrophage-derived Arg1 promotes tissue repair, resolution of inflammation, and restoration of tissue homeostasis. Additionally, our human data support the role of Arg1 in inflammatory joint diseases. Macrophages in the synovium of rheumatoid arthritis patients with an active disease are characterized by low Arg1, while macrophages in milder disease activity synovium are characterized by high level of Arg1. In summary, our data revealed that enhanced Arg1 can ameliorate arthritic joint inflammation.
In detail, Arg1 derived products are polyamines and proline. Polyamines support cell proliferation and downregulate pro-inflammatory cytokines production, while proline feeds collagen synthesis and deposition. Type I collagen is the most abundant bone matrix protein and a major constituent in mineralization of bone. Consequently, it is tempting to speculate that enhanced proline production could restore bone erosion, occurring during arthritis, by rebuilding bone structure through collagen. However, it is also possible that it restores the normal joint tissue architecture.
Does Arg1 lay the foundation for novel therapies for inflammatory bone diseases? Indeed, arginine supplementation is considered as an ergogenic aid for physically active subjects. However, Arg1-derived products also promote vascular fibrosis and thickening, resulting in arterial stiffening, a risk factor for cardiovascular diseases (https://www.ahajournals.org/doi/10.1161/ATVBAHA.108.183392). Thus, the advantageous and disadvantageous features of Arg1 are highly dependent on its content of activation and local rather than systemic treatment may be appropriate to avoid collateral damage due to excessive Arg1 activation. It would be of great interest to investigate the beneficial effect of arginine supplementation in terms of inflammatory bone diseases, such as rheumatoid arthritis. In summary, our findings suggest that Arg1-derived products may contribute to the initiation of a remission phase of rheumatoid arthritic joint inflammation.
Our findings are reported in the article entitled “Transcription factor Fra-1 targets arginase-1 to enhance macrophage-mediated inflammation in arthritis” in Journal of Clinical Investigation (https://www.jci.org/articles/view/96832). This work was conducted by Nicole Hannemann, Shan Cao, Daniel Eriksson, Anne Schnelzer, Jutta Jordan, Martin Eberhardt, Ulrike Schleicher, Jürgen Rech, Andreas Ramming, Steffen Uebe, Arif Ekici, Juan D. Cañete, Xiaoxiang Chen, Tobias Bäuerle, Julio Vera, Christian Bogdan, Georg Schett, and Aline Bozec from Friedrich-Alexander-University Erlangen-Nürnberg(FAU) and Universitätsklinikum Erlangen (Germany) and supported by the Deutsche Forschungsgemeinschaft(CRC1181, BO3811/1-6, BO3811/1-5, BO3811/1-7, BO3811/1-1), the Interdisciplinary Center for Clinical Research (IZKF) (grant D23 and A63), and the German Federal Ministry of Education and Research (BMBF) (e:Bio MelEVIR [031L0073A]). All authors would like to thank all staff and participants involved in this study.

Nicole Hannemann, PhD
Friedrich-Alexander-University Erlangen-Nürnberg (FAU) and Universitätsklinikum Erlangen, Germany