HUBLE METHODS
In vivo micro-computed tomography analysis of the mouse tibia
Introduction
This HubLE Method describes the protocol for quantitative in vivo trabecular bone analysis of mouse long bones using micro-computed tomography (micro-CT).
Materials
- in vivo mCT scanner
- Mouse holders/bed with monitoring
- Anaesthesia device
- Powerful computing equipment
- Tools for image registrationCalf
Methods [Update]
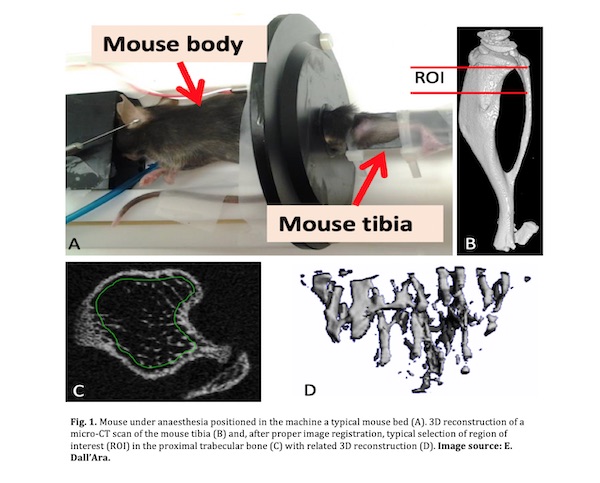
- Place the anaesthetized mouse in the scanner bed and secure the limb (Fig. 1, panel A) [Tip No. 1 and No. 2].
- Provide continuous anaesthesia during the scan and monitor breathing rate and mouse temperature [Tip No. 3].
- Set the X-ray source at a voltage of ~55kV, with a current of ~145mA. Also, optimise integration/exposure time [Tip No. 4].
- For scans of the whole or a portion of the tibia set the voxel size at ~10mm and the rotation step at 0.4–0.5° (number of projections at 720-900) [Tip No. 4].
- Select the region you would like to scan and start scanning [Tip No. 5 and No. 6].
- Once the scan has finished, remove mouse to a recovery chamber and reconstruct the images.
- After reconstruction, images can be registered with rigid image registration and analysed using an analysis software (Fig. 1, panel B) [Tip No. 7].
- Define a region of interest (ROI) in the proximal tibia metaphysis for trabecular bone analysis (Fig. 1, panel C), in the diaphysis for cortical bone analyses [Tip No. 8].
- Specify the ROI using a manual or semi-automatic drawing tool at different levels (Fig. 1, panel C).
- Apply noise reduction filter (e.g. Gaussian), perform global threshold and use a despeckling operator to remove unconnected speckles [Tip No. 9].
- Trabecular and Cortical output parameters are calculated using a 3D-analysis software [Tip No. 10].
- 3D-models of the ROIs can be visualised using a 3D visualization software (Fig. 1, panel D) [Tip No. 11].
Tips [Update]
Anaesthesia with isoflurane is adequate and the mouse should rest quietly with constant breathing rate and remain unresponsive to external stimuli, for example, absence of paw withdrawal reflex.
t is important to firmly secure the limb to prevent motion that causes blurring and double images. Tape, plastic ties and soft dental wax are radiolucent and will appear darker grey than the bone in the microCT image.
Under optimal isoflurane anaesthesia, typical respiratory rate ranges between 40-60 breaths per minute. A significant decline in body temperature occurs under anaesthesia. Heating should be applied during the scan to maintain the mouse temperature within 1°C of baseline measures.
The ionising radiation can affect the cell behaviour and therefore the bone structure in longitudinal studies. For every new application major effects of the radiation on the bone morphology should be evaluated by comparing in the end of the study the properties of the irradiated and not irradiated bone (e.g. you can use an integration time of 100ms for scans of the whole mouse tibia).
The optimisation of the size of the region to be scanned is fundamental for in vivo scans in order to minimise the scanning time and ionising radiation.
When selecting the region to scan remember to include some anatomical landmarks as the growth plate and/or the distal tibio-fibular joint for the standardization of the analysed regions. Ideally the whole tibia should be scanned but the effect of radiation should be verified.
- Rigid registration is important to evaluate the same region of interest over time and across different animals. This step is fundamental for minimising the potential error from the mismatch between the regions of interest analysed in the different images. Rigid registration can be performed with Scanco IPL, Bruker 3D.Suite or external software as Amira, MeVisLab, 3Dslicer, etc. Use Lanczos interpolation to reduce the effect of the image registration.
- Choose for all the bone scans the same ROI in the trabecular bone using as reference the growth plate (where the bridge formed across the bone breaks) and setting the beginning of the trabecular ROI 100mm distal to that reference. The cortical bone ROI should be in the diaphysis region such as at the midshaft, computed as middle section between the most proximal and most distal part of the tibia. Typical ROIS size: 1mm.
- For the analyses of in vivo microCT images it is fundamental to apply these steps in order to reduce the image noise.
- Main trabecular and cortical output parameters are trabecular bone volume fraction, trabecular thickness, trabecular separation, trabecular number, cortical area, cortical thickness and marrow area.
- Use for example CTAn software, IPL, mCT Ray, Amira, or 3Dslicer software for 3D visualisation.
References [Update]
Campbell GM, & Sophocleous A. Quantitative analysis of bone and soft tissue by micro-computed tomography: applications to ex vivo and in vivo studies. BoneKEy Reports, vol. 3, 2014.
van ‘t Hof RJ, Dall’Ara E. Analysis of Bone Architecture in Rodents Using Micro-Computed Tomography. Methods Mol Biol. 2019;1914:507-531.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, & Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone and Miner Res, 2010;25:1468–1486.
Lu Y, Boudiffa M, Dall’Ara E, Liu Y, Bellantuono I, Viceconti M. Longitudinal effects of Parathyroid Hormone treatment on morphological, densitometric and mechanical properties of mouse tibia. J Mech Behav Biomed Mater. 2017; 75:244-251.

Enrico Dall’Ara, PhD
Department of Oncology and Metabolism and Insigneo institute, University of Sheffield, UK
Email: e.dallara@sheffield.ac.uk

Mattia Capulli, PhD
Department of Oncology and Metabolism and Insigneo institute, University of Sheffield, UK
Email: b.c.roberts@sheffield.ac.uk

