IN A NUTSHELL
SLIT3: promoting bone formation by targeting the skeletal endothelium
Osteoporosis-associated skeletal fractures are common and deadly. 1 in 2 women and 1 in 4 men will experience an osteoporotic fracture during their lifetime, and these fractures kill as many women each year as breast cancer. One contributing factor in the inadequacy of current clinical approaches is the relative dearth of anabolic agents to promote new bone formation. In approaching this problem, many investigators have focused on identifying new agents that can enhance the cell intrinsic ability of osteoblasts to build bone. However, bone formation involves more than osteoblasts, also relying on several supporting cell types to enable bone formation.
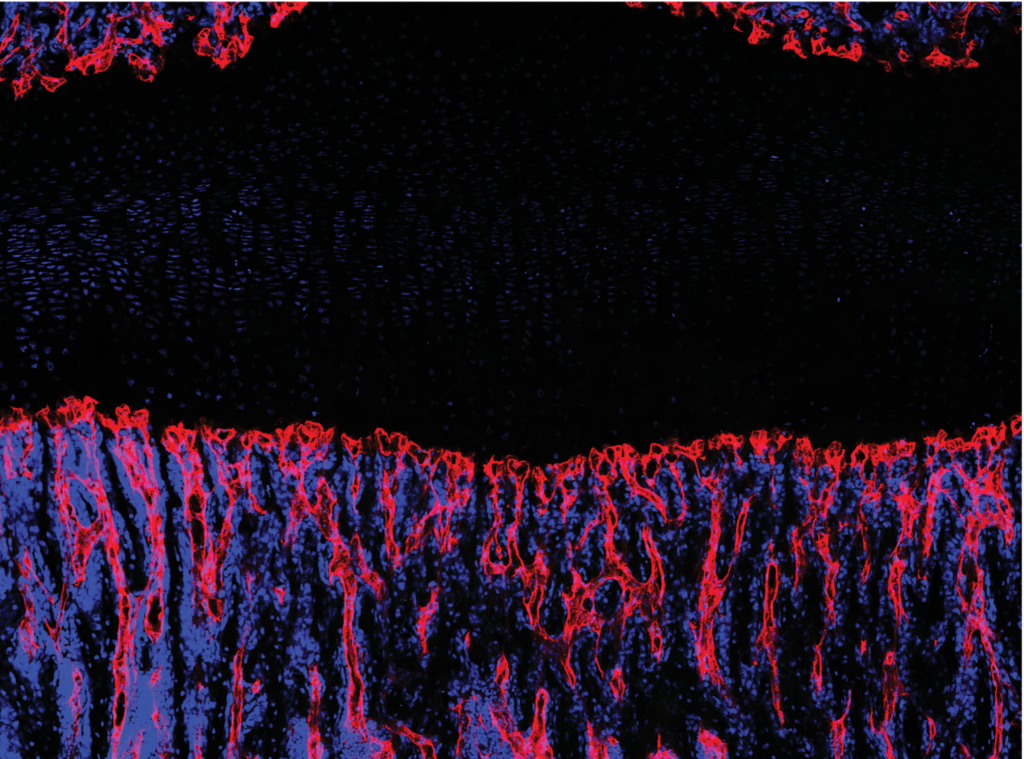
It is well appreciated that vascular endothelium runs throughout bone and is especially rich in regions of active bone formation, such as near the growth plate. Recent studies have identified a specialized subset of CD31hiEndomucinhi (EMCNhi) vascular endothelium that regulates bone formation. Further study showed that platelet-derived growth factor-BB (PDGF-BB) secreted by preosteoclasts induces CD31hiEmcnhi vessel formation during bone modeling and remodeling. However, it remains unclear how CD31hiEmcnhi endothelium levels are coupled to anabolic bone formation.
Osteoporosis-associated skeletal fractures are common and deadly. 1 in 2 women and 1 in 4 men will experience an osteoporotic fracture during their lifetime, and these fractures kill as many women each year as breast cancer. One contributing factor in the inadequacy of current clinical approaches is the relative dearth of anabolic agents to promote new bone formation. In approaching this problem, many investigators have focused on identifying new agents that can enhance the cell intrinsic ability of osteoblasts to build bone. However, bone formation involves more than osteoblasts, also relying on several supporting cell types to enable bone formation.
It is well appreciated that vascular endothelium runs throughout bone and is especially rich in regions of active bone formation, such as near the growth plate. Recent studies have identified a specialized subset of CD31hiEndomucinhi (EMCNhi) vascular endothelium that regulates bone formation. Further study showed that platelet-derived growth factor-BB (PDGF-BB) secreted by preosteoclasts induces CD31hiEmcnhi vessel formation during bone modeling and remodeling. However, it remains unclear how CD31hiEmcnhi endothelium levels are coupled to anabolic bone formation.
Our prior studies identified the adaptor protein Schnurri-3 (SHN3) encoded by the Hivep3 gene in human and mice as a potent endogenous inhibitor of bone formation. In our current study published in Nature Medicine, we used the greatly increased bone formation seen in mice with a conditional deletion of Shn3 to examine how osteoblasts shape their ancillary supporting tissues to support bone formation. Specifically, we demonstrated that not only does Shn3 regulate osteoblast differentiation, but it also regulates the ability of osteoblasts to promote the growth of skeletal CD31hiEmcnhi vascular endothelium. Expression analysis identified an axon-guidance cue, SLIT3, as a novel osteoblast-derived proangiogenic factor whose expression is negatively regulated by Shn3. Subsequent analysis of SLIT3-deficient mice or mice lacking the SLIT3 receptor ROBO1 demonstrated reduced CD31hiEmcnhi vascular endothelium and low bone mass due to impaired bone formation, and that SLIT3 is necessary for the high bone mass phenotype of Shn3-/- mice. This osteo-angio crosstalk pathway is also essential for bone healing, as shown by the complete blockade in fracture repair in SLIT3-deficient mice and enhanced fracture repair in Shn3-/- mice. Accordingly, drugs that target the SLIT3 pathway may represent a novel class of vascular-targeted osteoanabolic therapy as administration of recombinant SLIT3 both enhanced bone-fracture healing and counteracted bone loss in a mouse model of postmenopausal osteoporosis.
Collectively, these findings imply that SLIT3 production is a means for osteoblasts to condition their environment through angiogenesis to be conducive for bone formation. To our knowledge, SLIT3 also provides the first demonstration of a new class of vascular-targeted therapeutics in bone. This provides therapeutic proof-of-principle that the pool of cells that can be directly targeted to treat osteoporosis or promote fracture healing is not limited to osteoblasts and osteoclasts, but also extends to endothelial cells. The further identification of anabolic factors governing the endothelium-osteoblast crosstalk will provide combinatorial approaches to increase bone mass and improve bone regeneration.
These findings are described in the article entitled “Targeting skeletal endothelium to ameliorate bone loss” published in Nature Medicine. This work was conducted by Ren Xu (currently an independent principal investigator at School of Medicine, Xiamen University, China), Alisha Yallowitz, Dong Yeon Shin, Jung-Min Kim, Shawon Debnath, Sarfaraz Lalani, Na Li, Pouneh Kermani, Yifang Liu, Jason M. Butler, Annarita Di Lorenzo and Matthew B. Greenblatt from Weill Cornell Medical College in collaboration with An Qin, Zhuhao Wu, Gang Ji, Mathias P. Bostrom, Xu Yang, Chao Zhang, Han Dong, Michael G. Poulos, Amanda Wach, Yi Zhang, Kazuki Inoue, Baohong Zhao, Jae-Hyuck Shim, Laurie H. Glimcher from other institutes in China and US. This research was supported by Career Award for Medical Scientists from the Burroughs Wellcome Foundation and is funded by the Office of the Director of the NIH under award DP5OD021351 and a Junior Investigator Award from the Musculoskeletal Transplant Foundation. Authors would like to thank all staff and participants involved in this study.

Ren Xu, PhD
School of Medicine, Xiamen University, China.