The IDG-SW3 osteoblast-to-late-osteocyte cell line is derived from a temperature-sensitive DMP1-GFP transgenic mouse. IDG-SW3s cultured with interferon g (IFNγ) at 33oC will proliferate, whilst culture in mineralising conditions without IFNγ at 37oC enhances differentiation. This HubLE Method describes the protocol for culturing this cell line [1].
ProlifαMEM: Add 10% heat-inactivated foetal calf serum (FCS), AB/AM (100U/ml penicillin, 100mg/ml streptomycin, 0.25mg/ml amphotericin) and L-glutamine (200mM). Aliquot the stock media and add IFNγ (2500U/ µl). Incubate the medium at 33oC prior to use and limit exposure to heat due to IFNγ degradation.
OcyMEM: Add 10% heat-inactivated foetal calf serum (FCS), AB/AM (100U/ml penicillin, 100mg/ml streptomycin, 0.25mg/ml amphotericin) and L-glutamine (200mM). Add 50µg/ml ascorbate and 2-4mM β-glycerophosphate (the original paper uses 4mM β-GP [1], however, IDG-SW3 cells differentiate and mineralise sufficiently in 2mM). Always make fresh on the day of use.
Collagen-coated tissue culture plates/ flasks: Ensure all plates are coated under sterile conditions in a tissue culture hood. Use a cold pipette (stored in the freezer until use) to stop the collagen sticking to the plastic. The 0.15mg/ml collagen solution can be re-used 5-6 times; coating for ~1 hour each time. Coated plastics wrapped in parafilm can be stored at 4oC for up to 6 months until use.
Seeding density: IDG-SW3 cells will mineralise in 12 and 6-well plates but due to the long culture duration some monolayer peeling should be expected. Woo et al. [1] recommend seeding IDG-SW3 cells at 4×104 cells/cm2, although the lower densities of 104 (12-well) and 105 (6-well) will also support osteocyte proliferation, mineralisation and differentiation. To expand IDG-SW3 cells for the subsequent passage seed at 5×105 cells/ 75cm2 flask.
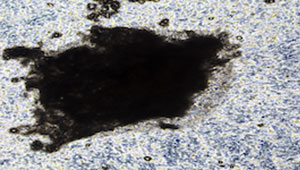
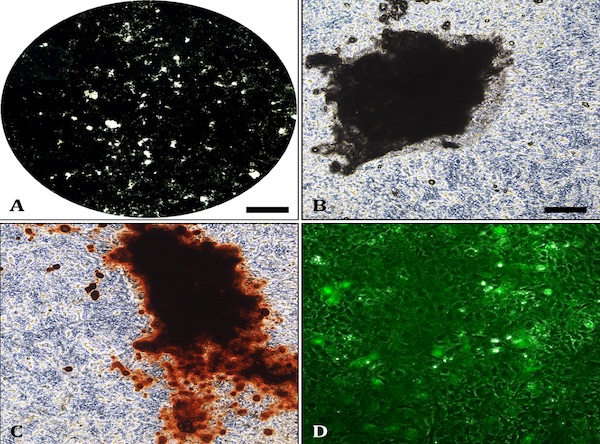
Alizarin Red staining: Mineralised bone nodules can be stained with alizarin red (Fig.1C). It is also possible to obtain good quality images on unstained cell layers (Fig 1A-1B). DMP1-GFP expression can be visually monitored throughout the differentiation process (Fig.1D). Evaluation of E11, DMP1 and sclerostin gene/ protein expression is also advisable to confirm osteocyte differentiation.